PATIENT FIRST
Do you or a loved one suffer from AL Amyloidosis?
Discover if you qualify for our clinical trial and take a proactive step towards potential groundbreaking treatment. Join us in advancing care and contributing to medical research that could change lives.
Voices of Hope: Real Stories from AL Amyloidosis Warriors
Discover the transformative journeys of individuals who have bravely navigated their treatment with our groundbreaking therapy. Hear firsthand how their lives have been changed and their futures reimagined.
BE PROACTIVE IN AL
Act Early, Live Better
Immix Biopharma’s ‘Be Proactive in AL’ initiative empowers patients and healthcare providers to recognize and address AL amyloidosis early. By enhancing awareness and promoting early diagnostic strategies, we aim to significantly improve patient outcomes and quality of life through timely interventions. Join us in transforming the approach to this challenging disease.
Revolutionizing Treatment: Our Patient-First Approach
At the heart of our mission lies a steadfast commitment to patient welfare. Our innovative single-day CAR-T therapy, the first of its kind, is designed to minimize disruption in our patients’ lives while maximizing treatment effectiveness. By reducing hospital stays and side effects, we ensure a smoother, more comfortable recovery process, prioritizing your health and time.
What is AL Amyloidosis?
AL Amyloidosis is a rare but serious disease that occurs when an abnormal protein called amyloid builds up in your organs and tissues. These proteins are produced in the bone marrow and can accumulate in different parts of the body, including the heart, kidneys, liver, spleen, nervous system, and digestive tract. This buildup can interfere with the normal function of these organs and can lead to severe health complications if left untreated.
Exploring New Frontiers with CAR T-Cell Therapy
At Immix Biopharma, we are pioneering a new approach to treating AL Amyloidosis using CAR T-cell therapy. This innovative treatment harnesses your own immune system to fight the disease, offering a potential curative solution where traditional treatments have failed. Early results have shown promising results, providing new hope for patients who have exhausted other options.
Learn More
If you or a loved one is battling AL Amyloidosis and standard treatments haven’t worked, it may be time to explore new options. Contact us to learn more about our CAR T-cell therapy and our current clinical trial.
BE PROACTIVE IN AL
Act Early, Live Better
U.S. observed prevalence of AL Amyloidosis estimated to reach 33,277 patients in 2024 according to according to Staron, et al Blood Cancer Journal. No FDA approved treatments available for relapsed/refractory AL Amyloidosis
Due to its nonspecific symptoms, such as fatigue, weight loss, and swelling, the condition is often misdiagnosed or detected in later stages.
Without effective treatment, the median survival rate for AL patients with advanced cardiac involvement can be less than a year.
GIVING PATIENTS HOPE
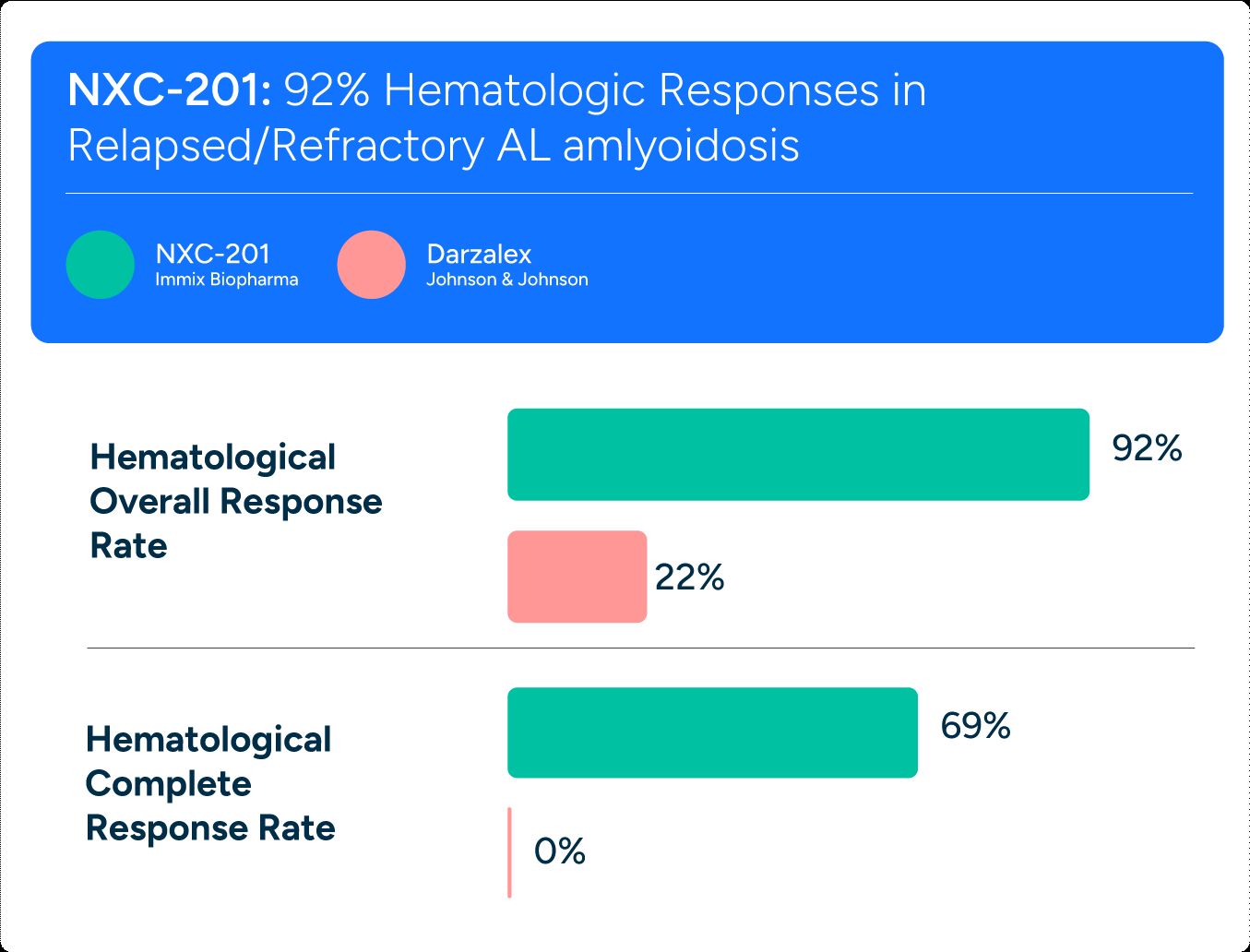
NXC-201
Only CAR-T in AL Amyloidosis
92% Overall Response rate in relapsed/refractory AL amloidosis.
(Median 4 lines of prior therapy prior to NX-201 – all including Darzalex)
Cardiac Response exceeds purpose-designed CAEL-101 and Birtamimab for relapsed/refractory patients
Zero Neurotoxicity of any grade in AL Amloidosis.
