INVEST IN THE FUTURE
AL AMYLOIDOSIS AND OTHER SERIOUS DISEASES CELL THERAPY CLINICAL TRAILBLAZER
Immix Biopharma is a clinical-stage biopharmaceutical company trailblazing cell therapies in AL Amyloidosis and select other serious diseases. Our lead candidate is sterically-optimized BCMA-targeted chimeric antigen receptor T (CAR-T) cell therapy NXC-201. NXC-201 is being evaluated in the U.S. Phase 1b/2a trial NEXICART-2 as well as the ex-U.S. study NEXICART-1.
WANT TO LEARN MORE?
Download our investor presentation to get all the latest information available.
RECENT EVENTS
RECENT NEWS
Immix Biopharma Attends FDA CEO Forum in Washington DC
– Selected to Attend In-Person Event on June 5 led by FDA Commissioner Marty A. Makary, M.D., M.P.H. – LOS...
Read MoreImmix Biopharma Announces Primary Endpoint Met in positive NXC-201 Interim Results Presented at ASCO, Enabling Path to Best-in-Class Therapy for relapsed/refractory AL Amyloidosis
Immix Biopharma today presented results at ASCO from its U.S. multi-center NEXICART-2 Phase 1/2 clinical trial of NXC-201, meeting its...
Read MoreImmix Biopharma Further Expands U.S. Clinical Sites for relapsed/refractory AL Amyloidosis Trial NEXICART-2
– Patient enrollment exceeding expectations – – 14 U.S. sites actively enrolling; 10 U.S. sites added since last update –...
Read MoreImmix Biopharma Announces Positive Results for NXC-201 at ASCO Oral Presentation, Enabling Pathway to Best-in-Class Therapy for relapsed/refractory AL Amyloidosis
Immix Biopharma today reported ASCO abstract results from its U.S. multi-center NEXICART-2 Phase 1/2 clinical trial of NXC-201 demonstrating strong...
Read MoreINVESTOR INFORMATION NAVIGATION
LET'S BUILD SOMETHING GREAT TOGETHER
Join our newsletter for exclusive insights into groundbreaking medical advancements, the latest in research, and transformative therapies. Stay informed and be part of shaping the future of healthcare. Sign up today!
CORPORATE HIGHLIGHTS
Initiates Patient Dosing in NEXICART-2 trial
Lead site Memorial Sloan Kettering Cancer Center (MSKCC) for US AL Amyloidosis NXC-201 clinical trial.
FDA APPROVAL OF IND APPLICATION FOR NXC-201
Opens up ability to treat patients in the US. Favorable tolerability enables potential expansion into other serious disease indications.
MULTIPLE SCIENTIFIC BOARD MEMBERS ADDED
Valuable expertise added from esteemed institutions, giving enhanced perspectives to our team
N-GENIUS CELL THERAPY PLATFORM
Possibility for additional cell therapies beyond current focus including possible expansion into other serious disease indications
FOUNDATIONAL EXCELLENCE
Paving the Way for Revolutionary Treatments
Comprehensive Clinical Foundation
Building on one of the largest clinical datasets in the cell therapy industry, Immix Biopharma has established a strong foothold in advancing cell therapies.
Pivotal Tolerability Milestone
Central to this achievement is the compelling evidence from a study involving 63 myeloma patients, pivotal in establishing the favorable tolerability profile of NXC-201, even when administered at high doses.
Stepping Stone to New Therapies
This foundational data serves as a critical stepping stone for exploring broader applications, providing a robust basis for venturing into new therapeutic areas.
Opportunity in AL Amyloidosis
Such a strong safety profile is especially significant as we turn our focus towards AL Amyloidosis—a condition with no approved therapies or other therapies in development.
Validating Future Directions
The initial positive outcomes from the first 13 AL patients further validate our approach and reinforce our commitment to expanding treatment horizons.
Expanding Treatment Horizons
Building on our success with AL Amyloidosis, we’re advancing our cell therapy technology to tackle a broad array of other serious diseases, extending our reach and impact.
WHERE WE ARE
By leveraging the established tolerability of NXC-201, Immix Biopharma is poised to offer new hope to patients suffering from AL Amyloidosis. The initial positive outcomes from the first AL patients treated further validate our approach and reinforce our commitment to expanding treatment horizons.
Memorial Sloan Kettering Cancer Center in NYC recently named as the lead clinical site for the NXC-201 relapsed/refractory AL Amyloidosis multi-site clinical trial.
On track to dose relapsed/refractory AL Amyloidosis patients with CAR-T NXC-201 at New York City lead site and other leading U.S. sites mid-2024
Patients Being Dosed
From December 2022 through December 2023, went from ~10 patients dosed with NXC-201 to 72 patients dosed
FDA Orphan Drug Designation
Received FDA Orphan Drug Designation for NXC-201 for AL Amyloidosis
FDA Orphan Drug Designation
Received FDA Orphan Drug Designation for NXC-201 for multiple myeloma
Publications & Presentations
Published in Haematologica, presented at the American Society for Gene and Cell Therapy, the International Myeloma Society, invited to present at the 65th American Society for Hematology
Scientific Advisor Additions
Memorial Sloan Kettering, Stanford, and Columbia University members joined Scientific Advisory Board
NXC-201 Produced for U.S. AL Amyloidosis Dosing
Produced 3 U.S. manufacturing batches of CAR-T NXC-201 for onboarding of U.S. clinical trials
NXC-201 U.S. First Patient Dosed
Dosed first patient in U.S. AL Amyloidosis clinical trial
BLUE OCEAN OPPORTUNITY
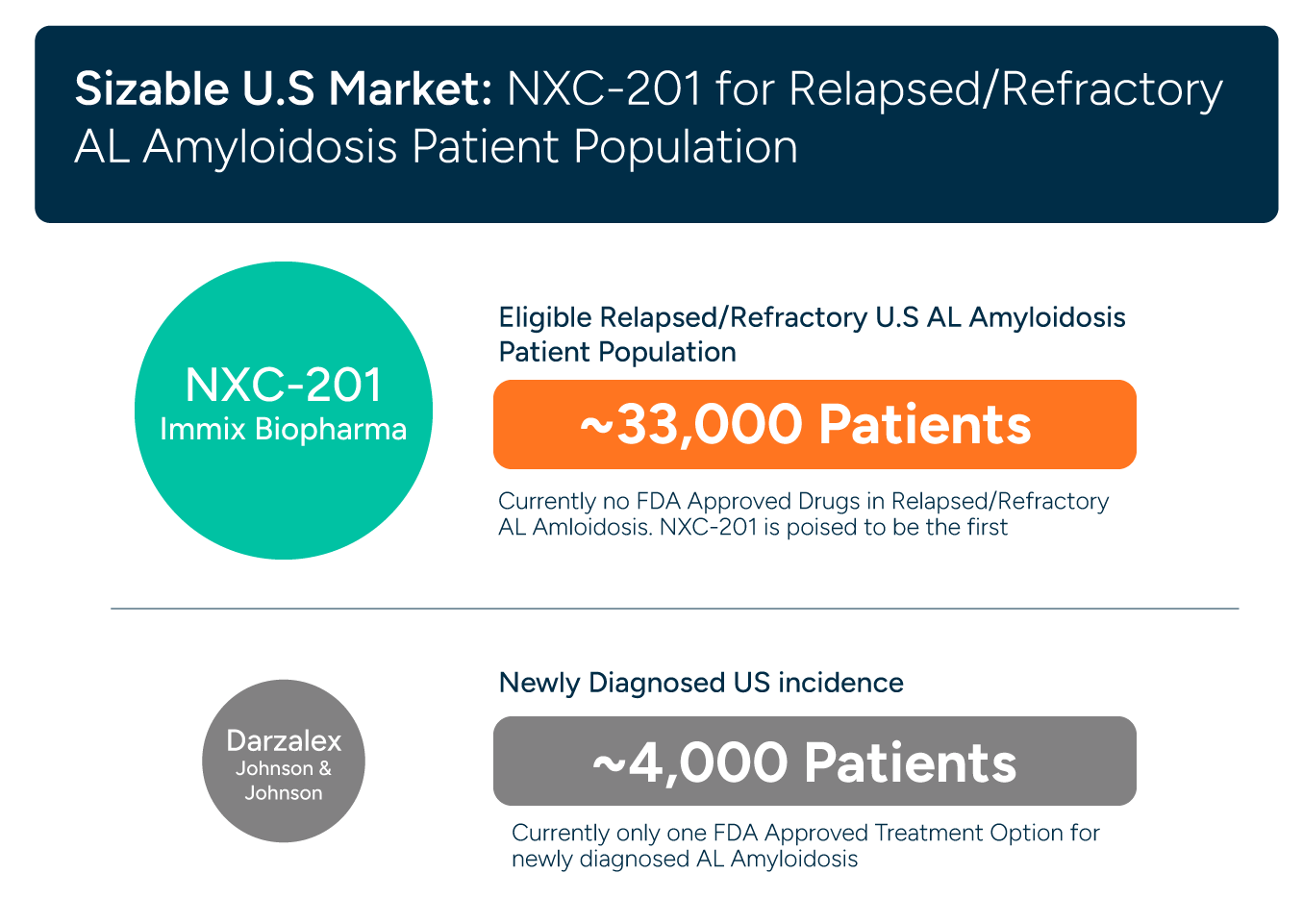
In relapsed/refractory AL Amyloidosis there are no drugs approved today (Amyloidosis is a $3 billion dollar market according to Grand View Research).
A BETTER SOLUTION
AL Amyloidosis: NXC-201 currently Phase 1b/2a clinical trial CAR-T NXC-201 in relapsed/refractory AL Amyloidosis: 92% overall response rate in clinical trials ($3B market)
Received FDA Orphan Drug Designation for NXC-201 for AL Amyloidosis. On Track to Dose NXC-201 Patients in United States.
AL AMYLOIDOSIS MARKET
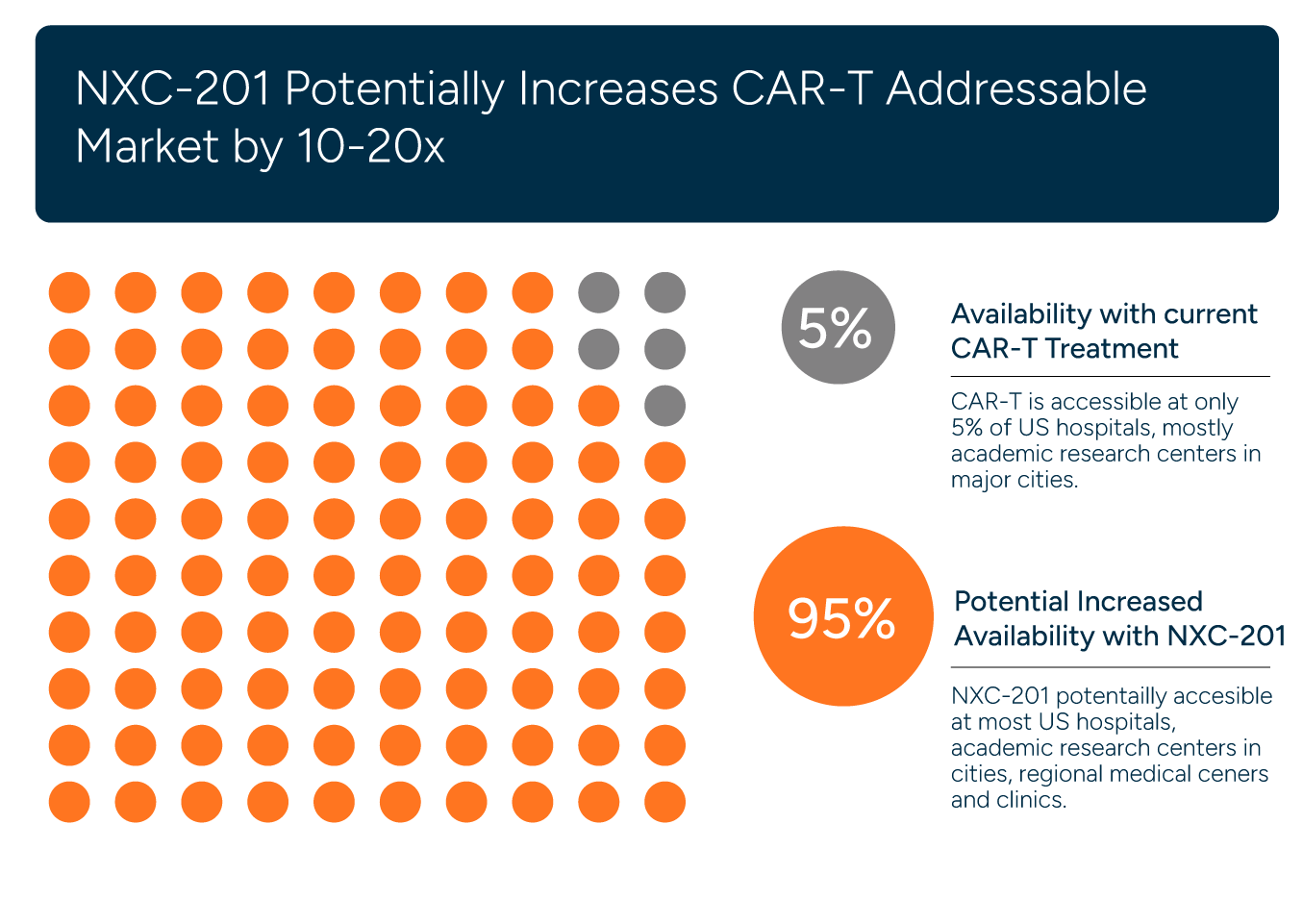
INDUSTRY LEADING PARTNERS
Immix Biopharma collaborates with top-tier industry leaders to accelerate our research and development efforts. Together, we are committed to advancing groundbreaking therapies that promise to transform patient outcomes in AL Amyloidosis and select other serious diseases.

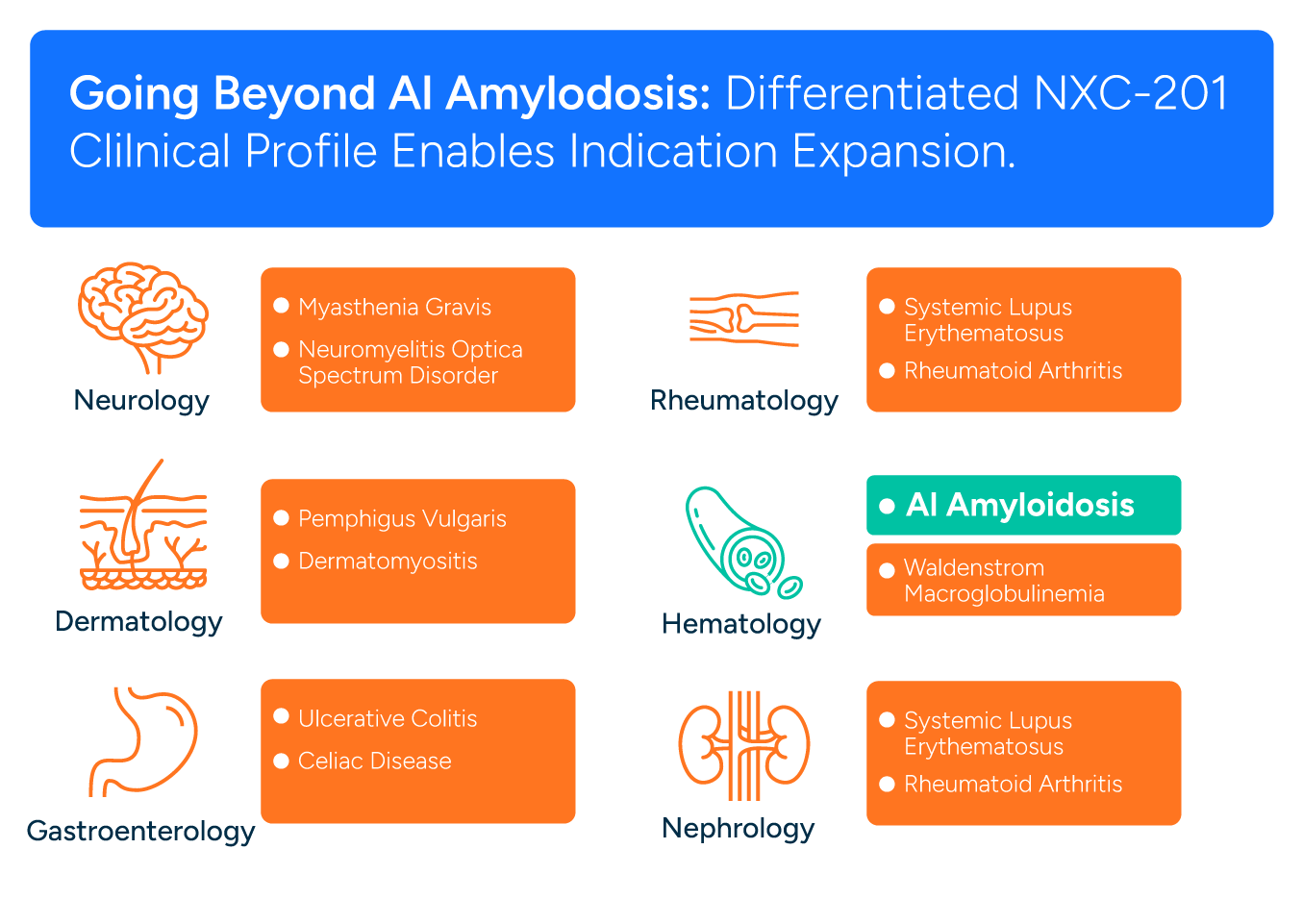
GOING BEYOND AL
STOCK INFORMATION
STOCK PRICE
STOCK CHART
HISTORICAL DATA
SEC FILINGS
GOVERNANCE
GOVERNANCE DOCUMENTS
CHARTERS
ANALYST COVERAGE
EDISON RESEARCH
Arron Aatkar, PhD
Jyoti Prakash, CFA
H.C. WAINWRIGHT
THINKEQUITY
INVESTORS FAQS
Our corporate headquarters are in Los Angeles, California.
Philadelphia Stock Transfer, Inc.
2320 Haverford Road, Suite 230,
Ardmore, PA 19003
(484) 416-3124
30 Rockefeller Plaza
New York, NY 10112
T: +1.212.653.8700
F: +1.212.653.8701

