CAR-T Clinical Trailblazer in AL Amyloidosis and autoimmune diseases
Stay up to date on the latest news alerts from immixBio
Stock Data
Latest News
Nasdaq: IMMX
Recent Events
Investor Presentation
The Problem
- In relapsed/refractory AL Amyloidosis there are no drugs approved today
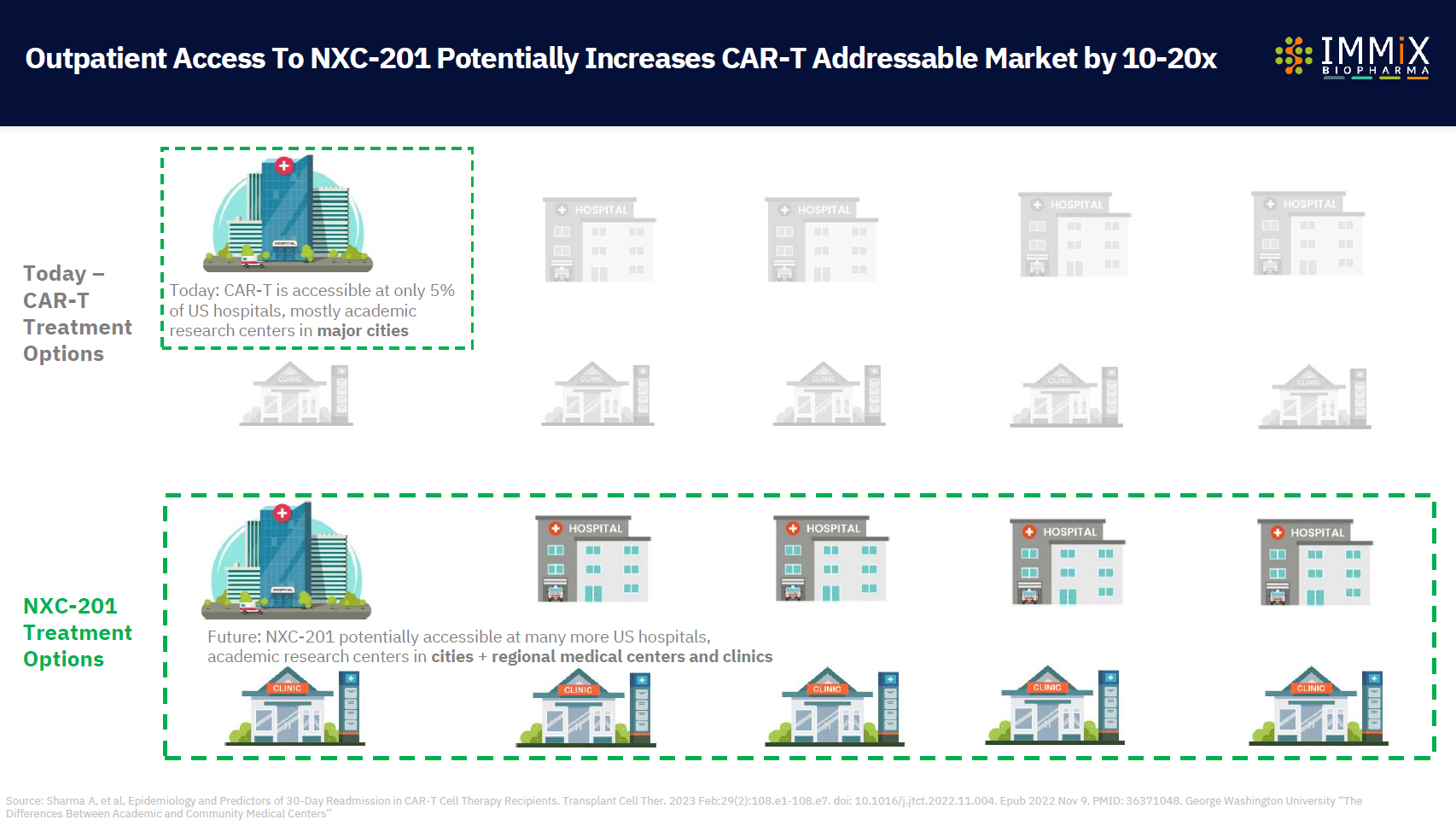
- In multiple myeloma, life-saving CAR-T cell therapies are only available in 5% of U.S. hospitals, and only 25% of patients on waiting lists actually receive the CAR-T
- In 95% of relapsed/refractory colorectal cancer patients, the median expected life span is just 5 months
The ImmixBio solution
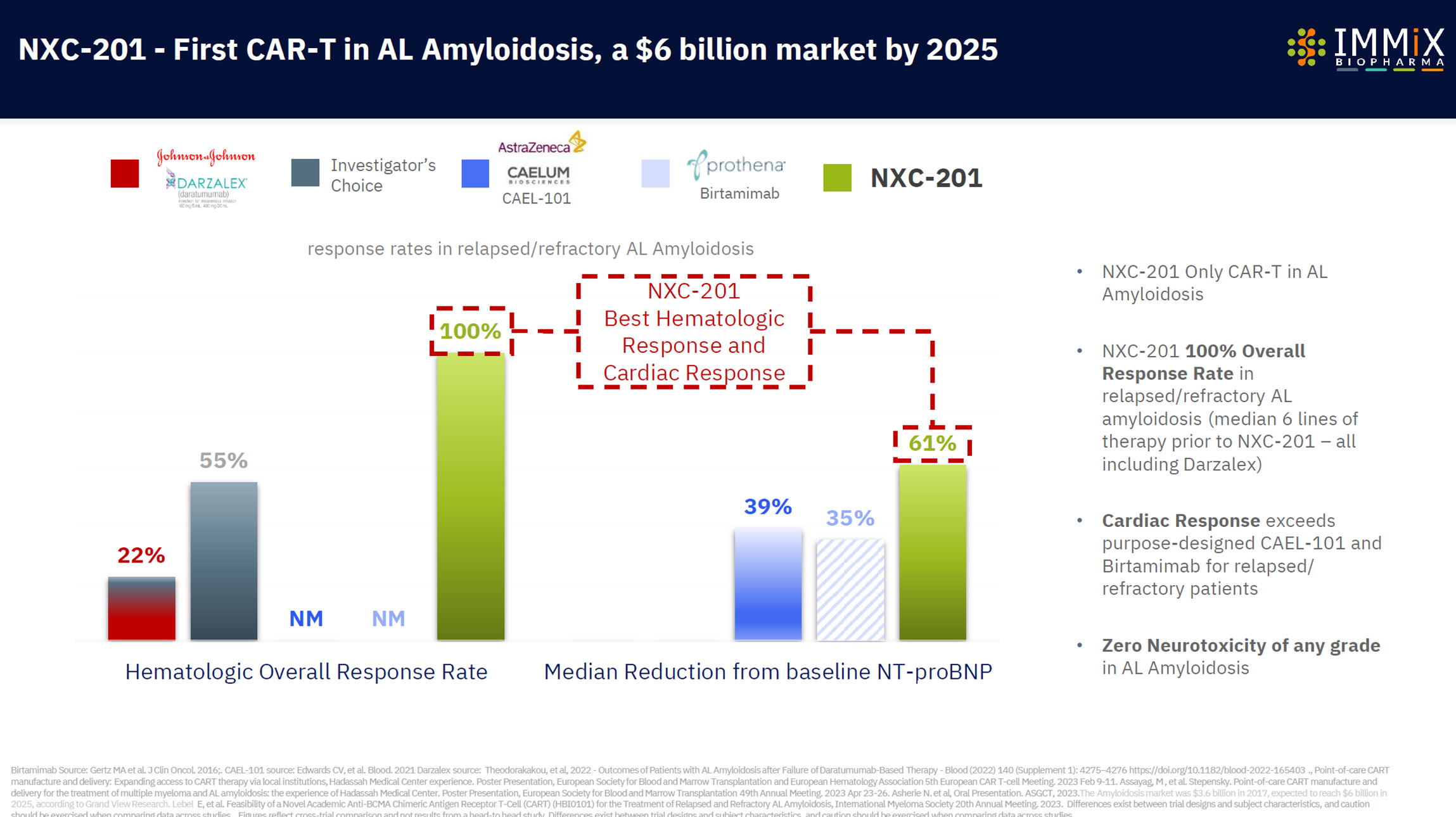
- CAR-T NXC-201 in relapsed/refractory AL Amyloidosis: 100% overall response rate in clinical trials ($3bn market)
- CAR-T NXC-201 in multiple myeloma: potential access in >90% of U.S. Hospitals ($18bn market)
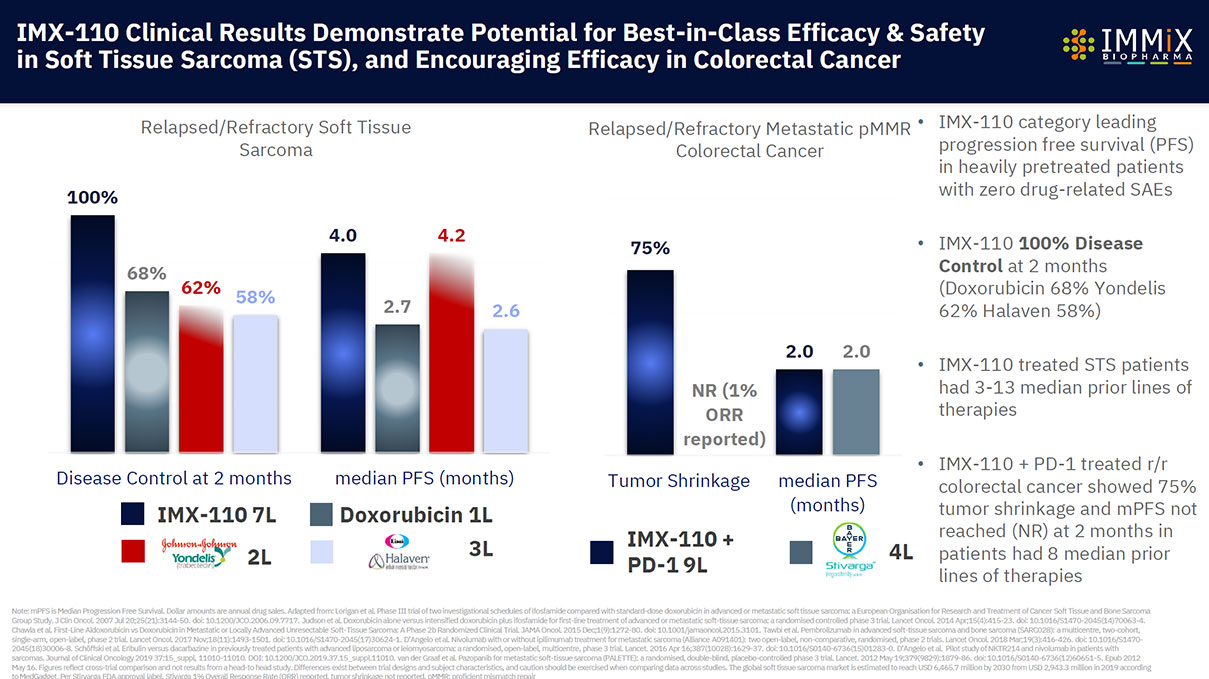
- IMX-110 in relapsed/refractory colorectal cancer: 75% tumor shrinkage observed at low dose in dose escalation study ($26bn market)
- AL Amyloidosis: NXC-201 currently Phase 1b/2a clinical trial
- 100% overall response rate
- 40 patients required to submit a BLA application to the FDA for approval
- Have dosed 9 NXC-201 patients so far (as of Sep 2023)
- Multiple Myeloma: NXC-201 currently Phase 1b/2a clinical trial
- 95% overall response rate
- 100 patients required to submit a BLA application to the FDA for approval
- Have dosed 63 NXC-201 patients so far (as of July 2023)
Recent Progress
- From Dec 2022 through Dec 2023, from ~10 patients dosed with NXC-201 to 72 patients dosed
- Received FDA Orphan Drug Designation for NXC-201 for AL Amyloidosis
- Received FDA Orphan Drug Designation for NXC-201 for multiple myeloma
- Published in Haematologica, presented at the American Society for Gene and Cell Therapy, the International Myeloma Society, invited to present at the 65th American Society for Hematology
- Memorial Sloan Kettering, Stanford, Columbia University members joined Scientific Advisory Board
- Produced 3 U.S. manufacturing batches of CAR-T NXC-201 for U.S. onboarding of U.S. clinical trials
Upcoming Catalysts
|
NXC-201
|
IMX-110
Other Candidates
2H23 – Finish preclinical studies with IMX-120 |
NXC-201
|
IMX-110
Other Candidates
2H23 – Finish preclinical studies with IMX-120 |
What is CAR-T Therapy
Each of our bodies has an immune system that recognizes and eliminates disease on a daily basis. When AL Amyloidosis or multiple myeloma emerges from our own body, however, the diseased cells are able to evade the immune system.
So how do we allow the body to recognize the diseased cells?
We believe the answer is NXC-201 CAR-T cell therapy.
CAR-T therapy, or chimeric antigen receptor T-cell therapy, is a type of immunotherapy that uses the patient’s own immune cells, modified with ImmixBio proprietary technology, to create NXC-201, which is then introduced into the patient’s body.
Then the patient’s modified NXC-201 CAR-T cells are able to recognize and eliminate diseased cells, with up to a 100% rate of disease reduction (overall response rate) in the case of relapsed/refractory AL Amyloidosis, or a 95% disease reduction (overall response rate) in the case of relapsed/refractory multiple myeloma.
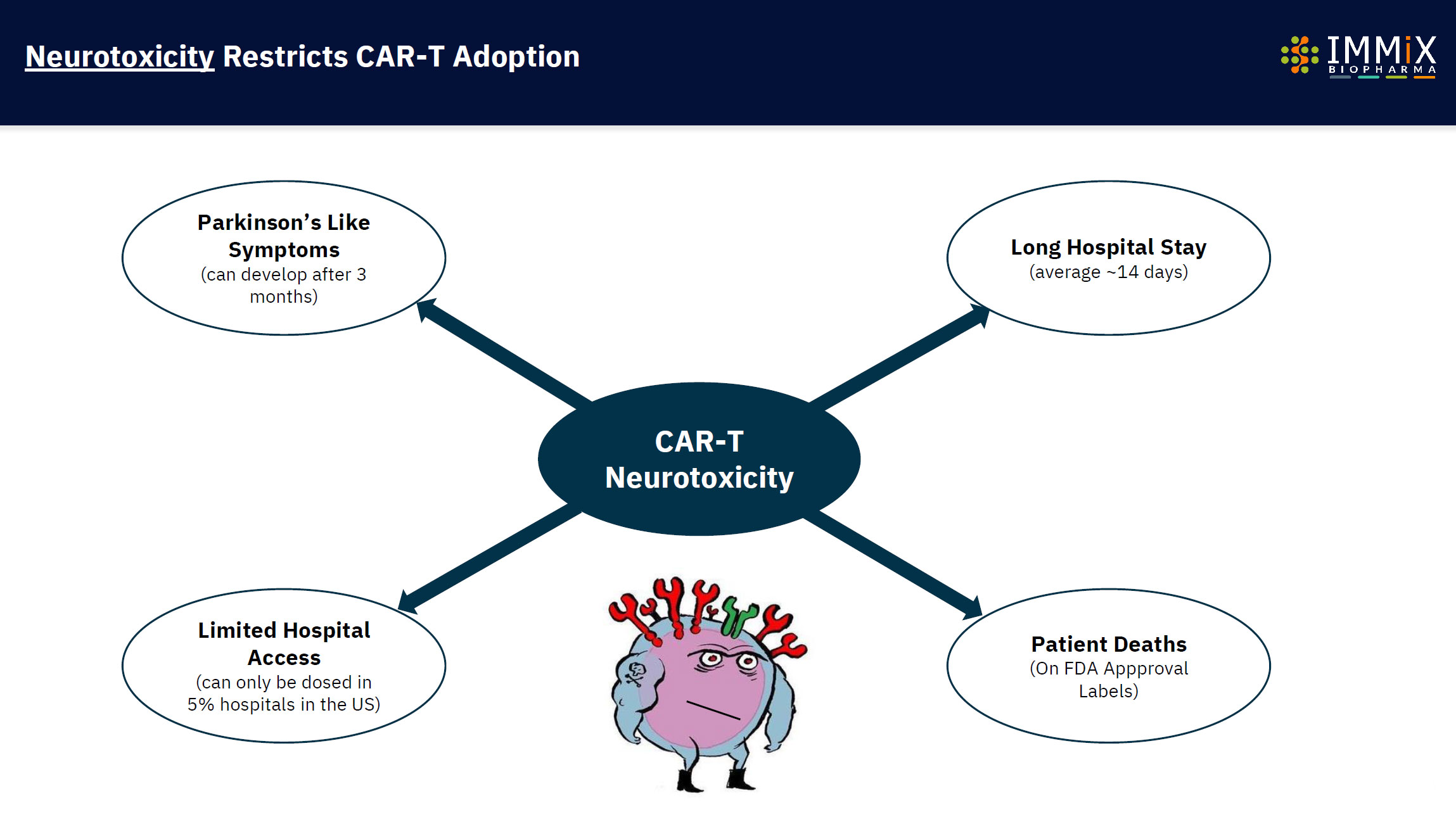
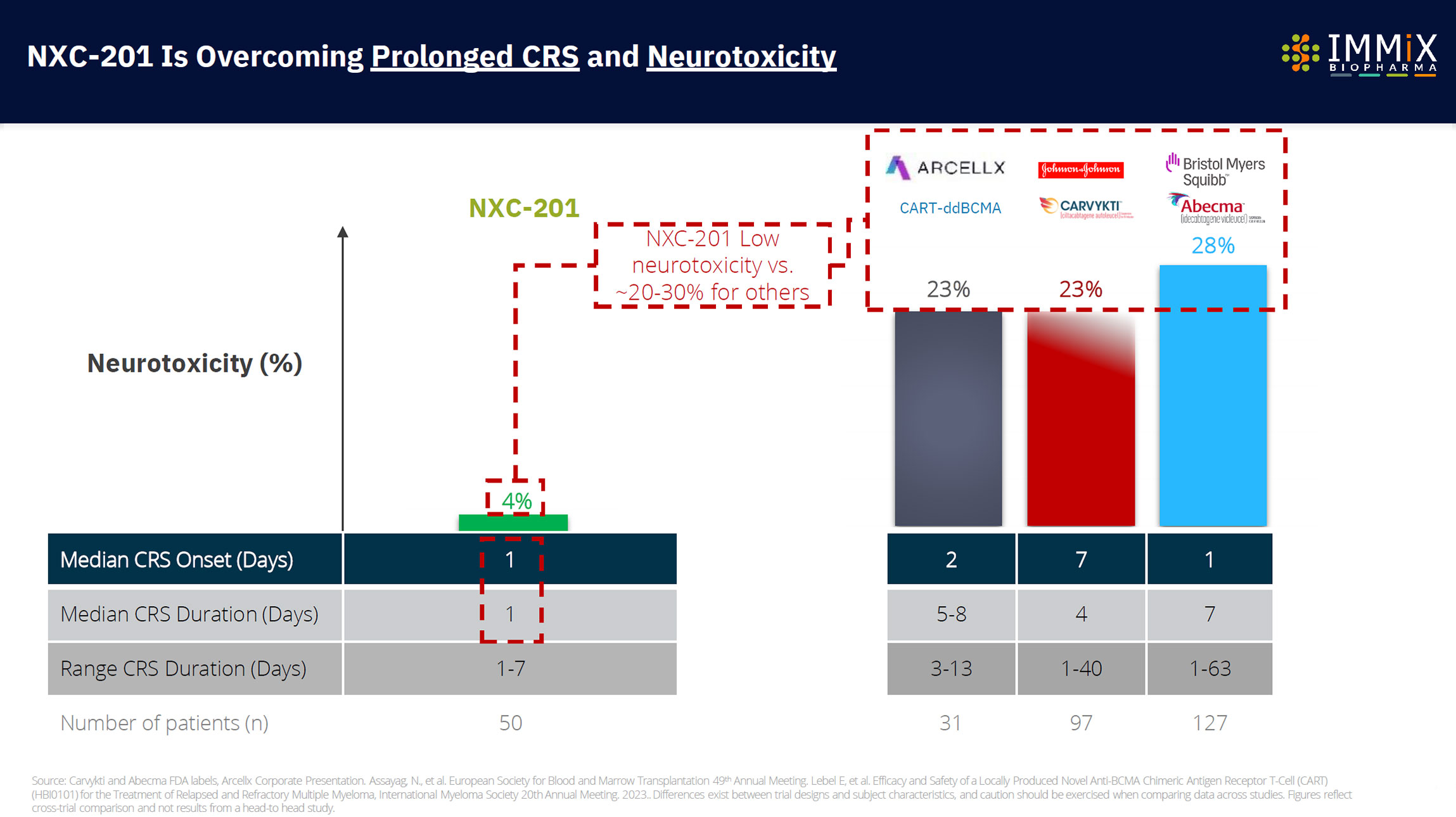
CAR-Ts in the market are only available in 5% of US Hospitals and produce up to 63-day duration side effects (cytokine release syndrome) and up to 578 days of neurotoxicity.
What makes us unique
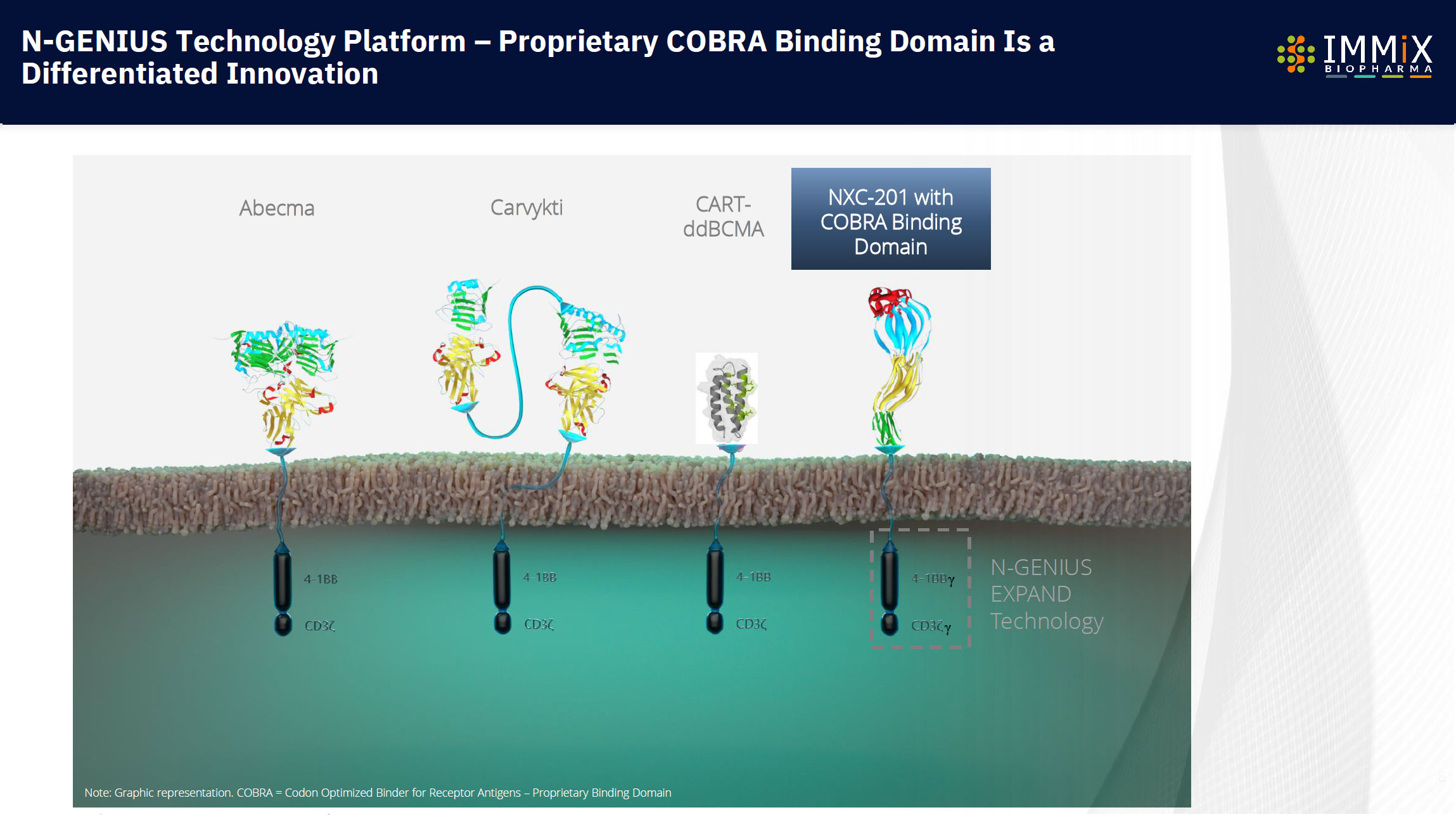
NXC-201 is the only CAR-T in AL Amyloidosis today, expanding into additional autoimmune indications, with a 100% overall response rate observed.
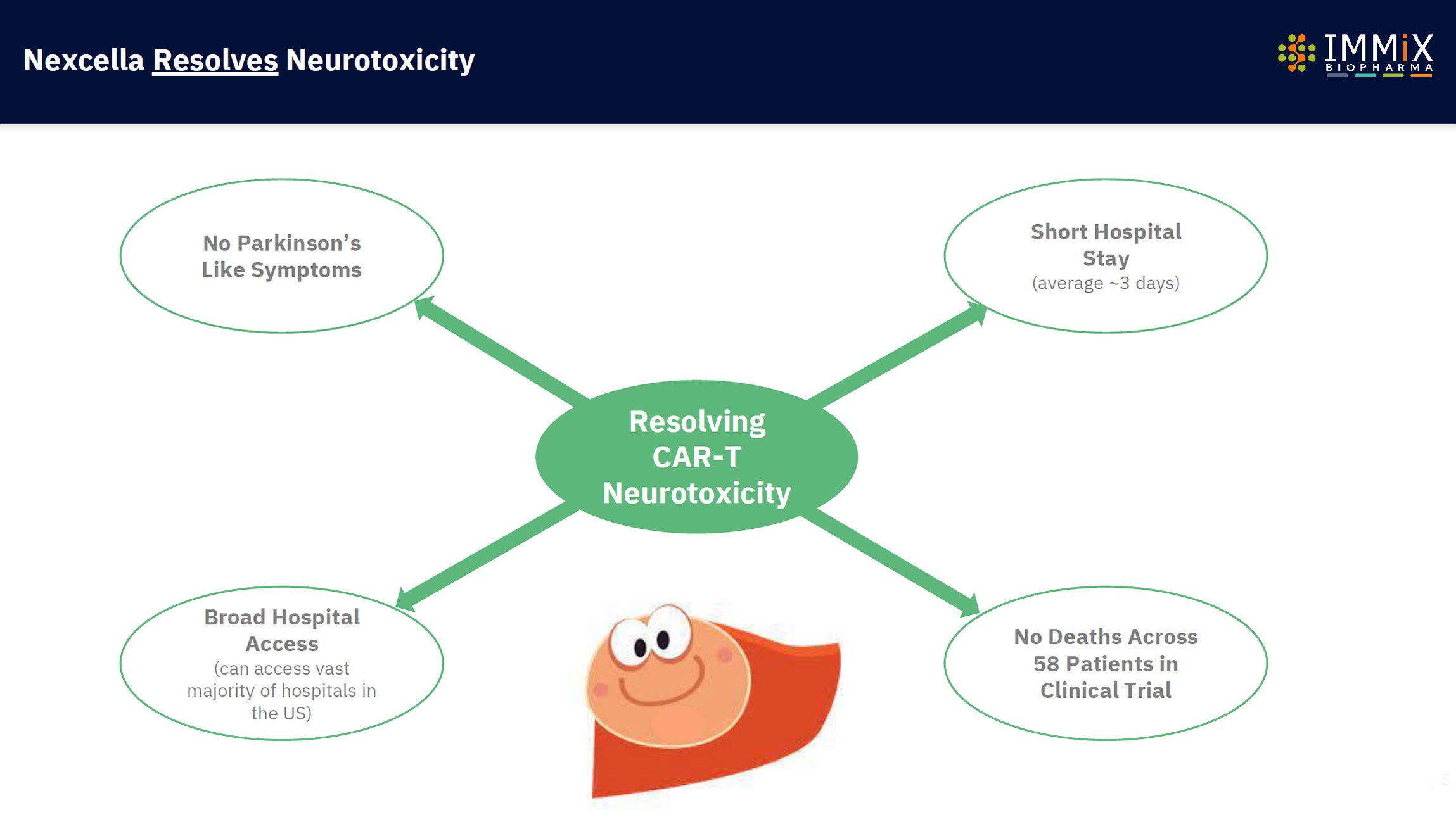
NXC-201 is the only CAR-T overcoming neurotoxicity.
NXC-201 is the only CAR-T that could be available in >90% of U.S. hospitals today
Team

Our executive team hails from UCLA, Goldman Sachs, Roche, AstraZeneca and GSK, and has a proven track record working together.
Our board members are former senior executives of Pfizer, BioMarin, as well as hedge fund and financial experts, and have been board members during many recent biopharmaceutical acquisitions, and Prometheus/Merck ($11bn); ChemoCentryx/Amgen ($4bn); Sierra/GSK ($2bn); Tobira/AbbVie ($2bn)
Harvard, Memorial Sloan Kettering, Stanford members comprise our scientific advisory board.
NXC-201 is the first CAR-T that we will be accessible by >90% of U.S. Hospitals, not just the 5% of U.S. hospitals that are able to offer CAR-T today
Our lead candidate is ImmixBio next-generation CAR-T cell therapy NXC-201 targeting:
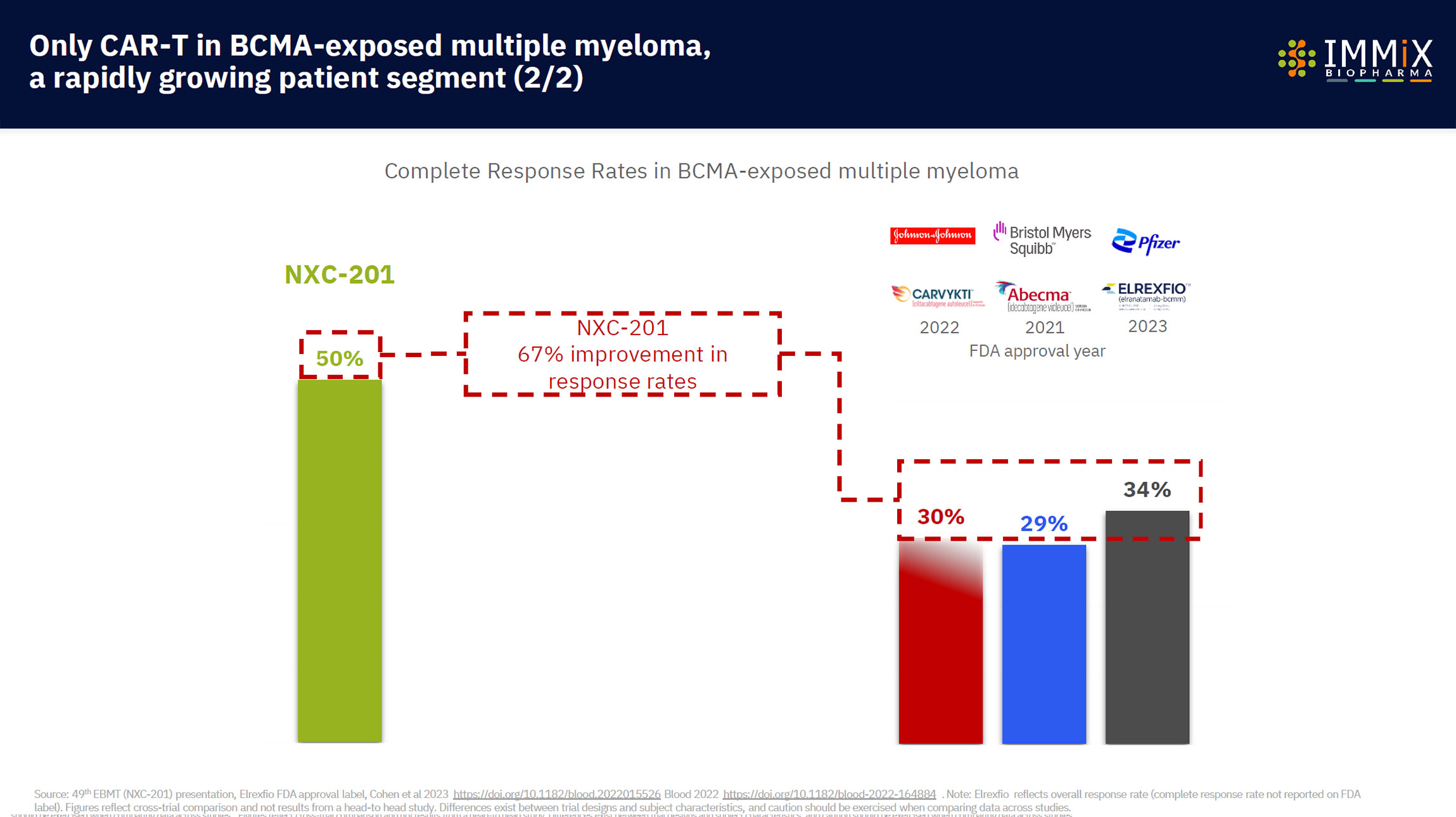
- Multiple Myeloma (63 patients treated) – 95% Overall Response Rate
- AL Amyloidosis (9 patients treated) – 100% Overall Response Rate
- $28.7 billion Multiple Myeloma market size expected to be reached in 2027 according to Wilcock, et al. Nature Reviews.
- $6 billion Amyloidosis market is expected to be reached in 2025, according to Grand View Research.
Differentiated tolerability profile with side effects lasting a fraction of competitors (median 1 day)
Other companies developing therapies in AL Amyloidosis include Prothena (Nasdaq:PRTA), and Caelum Biosciences CAEL-101 (part of AstraZeneca). Approved drugs include Darzalex/daratumumab (Johnson & Johnson)
ImmixBio tissue specific therapeutic IMX-110 targeting:
- Advanced Solid Tumors + Soft Tissue Sarcoma (30 patients treated)
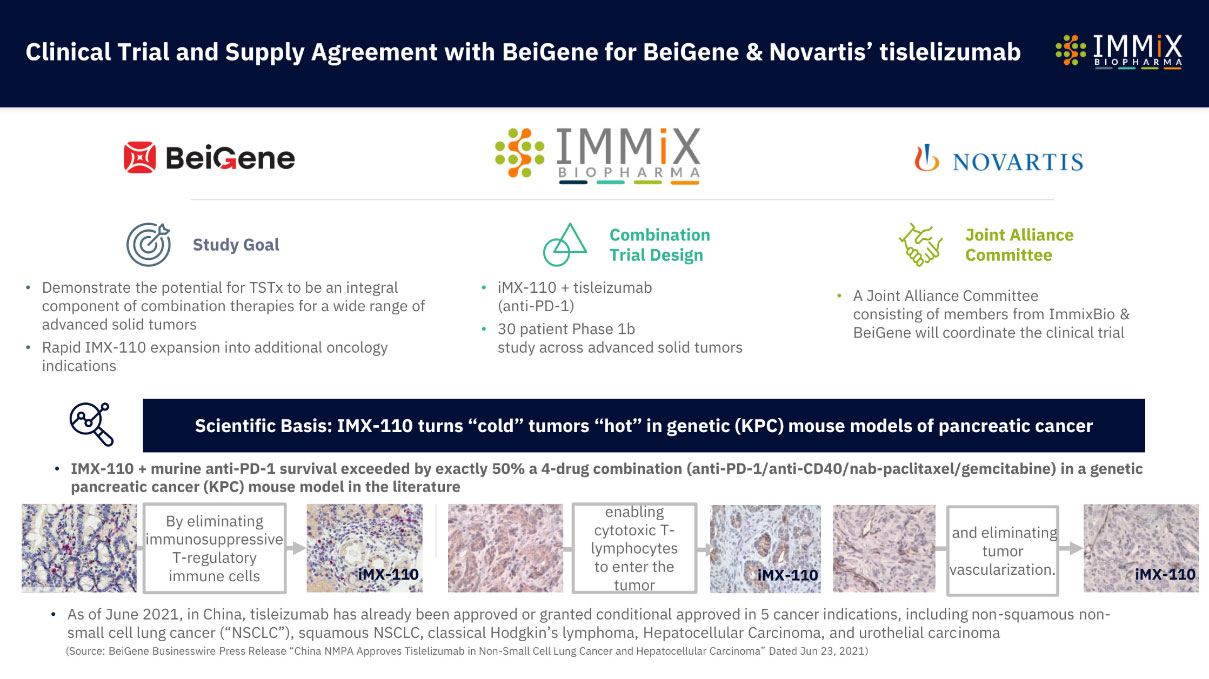
- Combination Clinical Trial IMX-110 + Beigene/Novartis’s anti-PD-1 tisleilizumab
- $6.5 billion soft tissue sarcoma market size expected to be reached in 2030
- $29 billion is the total combined sales of Keytruda (Merck) and Opdivo (Bristol Myers Squibb) – drugs targeting PD-1 in the market today according 2022 annual reports
- Combination Clinical Trial IMX-110 + Beigene/Novartis’s anti-PD-1 tisleilizumab ongoing in relapsed/refractory solid tumors
- Monotherapy IMX-110 Clinical Trial ongoing
- Clinical readouts expected on a rolling basis in 2023
Mission
ImmixBio’s mission is to harness the immune system through innovative cell therapies and other modalities to deliver widely accessible cures. Patients are waiting!