IMMIXBIO N-GENIUS CELL THERAPY PLATFORM
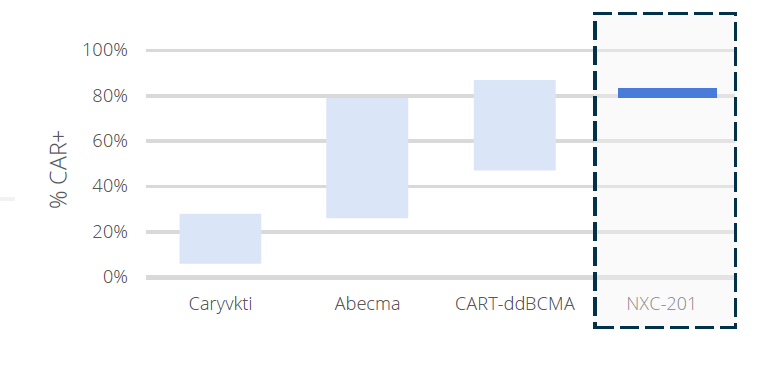
Our N-GENIUS platform, with EXPAND technology, has produced our pioneering lead candidate, CAR-T NXC-201, which has demonstrated an enhanced tolerability profile with the potential to reduce hospitalizations.
Our N-GENIUS platform enables our CAR-Ts to have distinct advantages including: preliminary clinical data — high overall response rate and durable responses; differentiated tolerability profile, resulting in a potential market first “Outpatient CAR-T” in NXC-201; and opportunity to treat a broader group of cancer patients.
Our N-GENIUS cell therapy platform, which has produced NXC-201, consists of three key elements: (1) purpose-built cell therapy evidence capture engine + relational database, which relates ImmixBio internal data to external to accelerate therapy design, manufacture, and preclinical; (2) proprietary EXPAND technology, which is applied to multiple cell therapy indications, already utilized to create NXC-201, to potentially increase efficacy and tolerability; and (3) atomized, novel binding scaffold generation engine, which allows us to make the correct binding for every molecule. We believe key characteristics of NXC-201 may apply to other products candidates produced by the N-GENIUS Platform. Those 3 key characteristics are: (a) high transduction efficiency (lower dose may lead to lower toxicity), (b) low tonic signaling (lower off-target toxicity may lead to lower toxicity), and (c) anti-exhaustion capability (increased persistence may lead to efficacy over an extended period of time).
N-GENIUS PLATFORM
3 KEY ELEMENTS
Purpose-Built Cell Therapy Evidence Capture Engine + Relational Database
Relating Nexcella internal data to external to accelerate therapy design, manufacture, and preclinical
Proprietary EXPAND technology
Applied to multiple cell therapy indications, already utilized to create NXC-201, to potentially increase efficacy and tolerability
Atomized, Novel Binding Scaffold
Generation Engine
Allows us to make the correct binding for every molecule
PRODUCED NXC-201
KEY CHARACTERISTICS
High Transduction Efficiency
(Lower dose may lead to lower toxicity)
*Carvykti data presented at ASH 2019; Abecma data presented at ASH 2017. CART-ddBCMA source Arcellrx
Low Tonic Signaling
(Lower off-target toxicity may lead to lower toxicity)
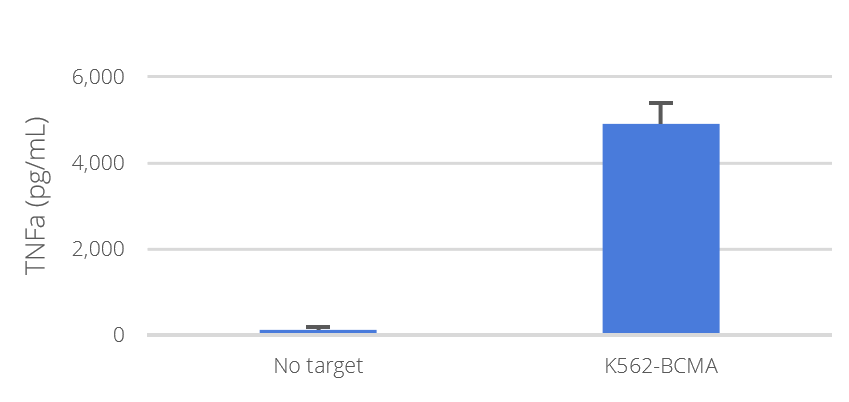
NXC-201 was co-cultured with the indicated target T cells and TNFα (B) and IL-2 (C) concentrations secreted in the culture supernatant were determined by ELISA.
Anti-Exhaustion Capability
(Increased Persistence may lead to efficacy over an extended period of time)
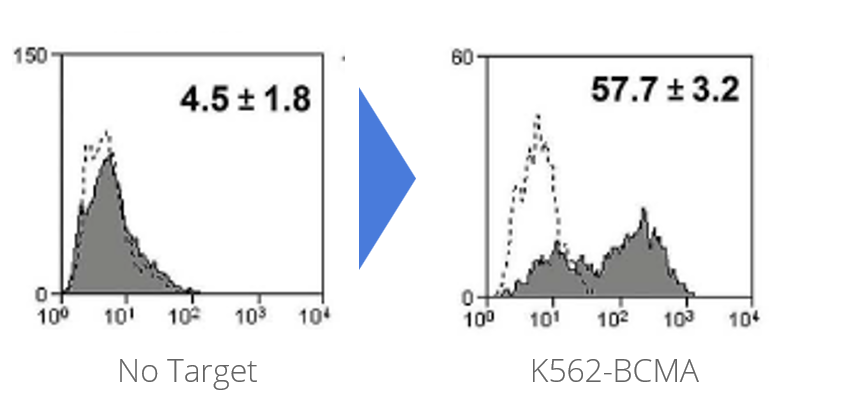
NXC-201 was co-cultured overnight then analyzed by flow cytometry for the expression of 4-1BB